Japanese researchers have created a dynamic microfluidic channel that vastly enhances the efficiency of circulate cytometers.
Circulate cytometry has enabled many breakthroughs in drugs and drug discovery. The approach examines single cells by detecting the fluorescence from their chemical tags because the cells cross by a laser beam. Most devices embrace a microfluidic channel, a slim passage that regulates the circulate of those tagged analytes. As a result of it permits fast counting and evaluation on the single-cell stage, circulate cytometry has turn out to be a cornerstone of recent biomedical analysis.
A strong various, impedance circulate cytometry, replaces the laser with electrodes that sense adjustments in electrical impedance (the full resistance {of electrical} gear to alternating present) as cells or particles transfer by the microfluidic channel. This method removes the necessity for fluorescent tags, which are sometimes costly and time-consuming to make use of. Nonetheless, its sensitivity might be restricted and its readouts inconsistent, for the reason that distance between flowing cells and the electrodes varies with channel top and particle measurement.
A New Adaptive Answer from NAIST
To fill this hole, a analysis staff led by Affiliate Professor Yalikun Yaxiaer from Nara Institute of Science and Know-how (NAIST), Japan, developed an revolutionary low-cost platform to beat these limitations. Their paper, revealed within the journal Lab on a Chip, was co-authored by Mr. Trisna Julian, Dr. Naomi Tanga, Professor Yoichiroh Hosokawa from NAIST, and others.
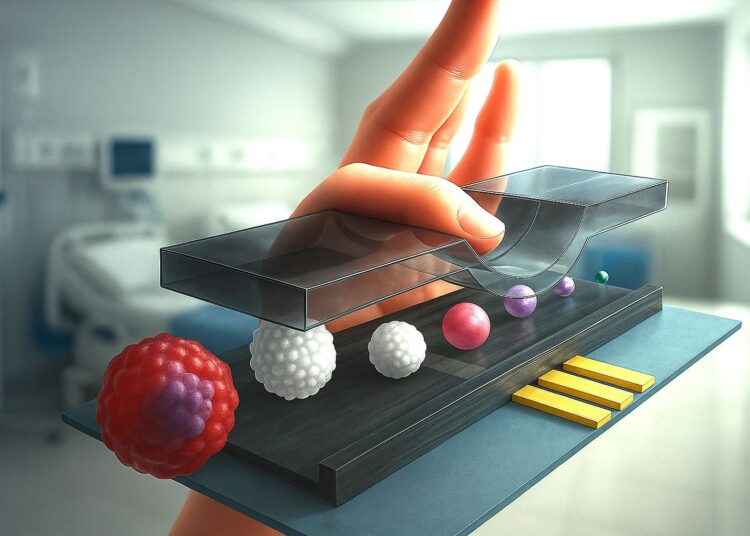
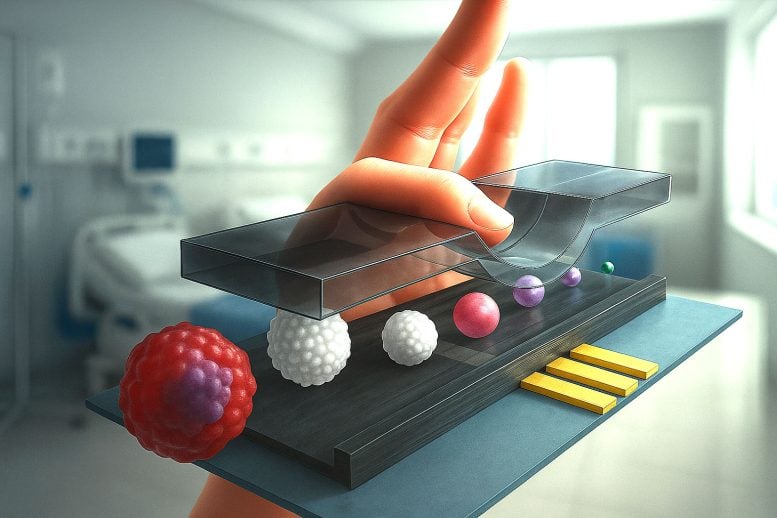
The staff’s design purpose was easy; they aimed to dynamically regulate the channel top relying on particle measurement. They realized this by attaching a metallic probe to the vertical axis of an XYZ stage—a laboratory gadget that permits extremely exact actions in three dimensions. By controlling the vertical place of the probe, they used its skinny tip to press in opposition to the highest of the 30-micrometer-high microfluidic channel of the circulate cytometer. This compression squeezed the channel barely, altering its top on demand.
By way of experiments and simulations, the staff confirmed that enabling the flowing particles to journey nearer to the sensing electrodes by decreasing the channel’s top led to a exceptional enhance within the platform’s sensitivity and accuracy. They achieved a three-fold amplification of the impedance signal by reducing the channel height by one-third, while also reducing the signal variability to half, allowing them to easily distinguish between multiple cells of different sizes.
Turning Clogging into an Advantage
Notably, by introducing a camera and an object-detection algorithm, the researchers found a way to leverage clogging (unwanted deposition of particles that prevents further passage of analytes) as a strategy to optimize the platform’s performance. “Our system deliberately induces a critical constriction by deforming the channel to maximize sensitivity. However, this deformation can be released just before actual clogging occurs,” explains Dr. Yaxiaer. “Thus, our approach acts like a smart microchannel that harnesses and controls the clogging phenomena.”
Overall, this study establishes a much-needed foundation for the standardization of adaptive impedance flow cytometry, paving the way for its integration into clinical and research contexts where precise cell analyses are required.
“Our findings underscore the potential for a universal, high-performance impedance flow cytometry platform—one that is simple, clog-resistant, and adaptable for a wide range of biomedical applications,” concludes Dr. Yaxiaer. Collaborating with medical institutions could transform this innovative platform into a diagnostic device for point-of-care testing, and could also be leveraged for drug development and testing.
Reference: “A long-term universal impedance flow cytometry platform empowered by adaptive channel height and real-time clogging-release strategy” by Trisna Julian, Tao Tang, Naomi Tanga, Yang Yang, Yoichiroh Hosokawa and Yaxiaer Yalikun, 26 August 2025, Lab on a Chip.
DOI: 10.1039/D5LC00673B
Never miss a breakthrough: Join the SciTechDaily newsletter.