A newly found hyperlink between pancreatic most cancers and neural signaling reveals a promising drug goal that slows tumor progress by blocking glutamate uptake.
Pancreatic most cancers is among the many most threatening cancers, and scientists are nonetheless working to grasp what drives its aggressive conduct. A analysis workforce on the Technical University of Munich (TUM) has recognized a organic mechanism that helps clarify this lethality.
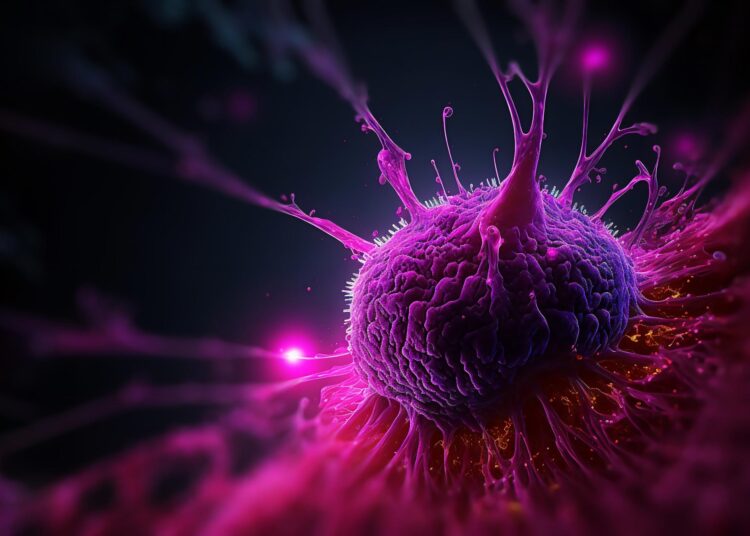
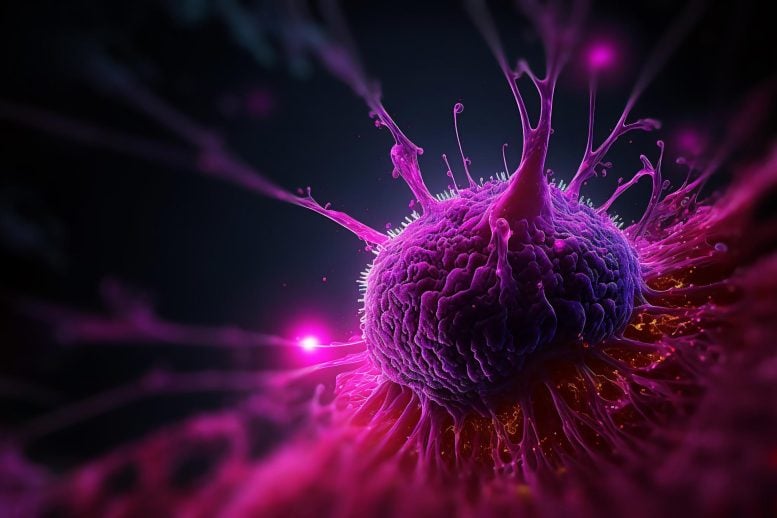
Their findings present that pancreatic most cancers cells can benefit from the physique’s nervous system by forming constructions known as pseudosynapses. Utilizing a selected receptor, these tumor cells take up the neurotransmitter glutamate, which then promotes their growth. The researchers believe this discovery could point to new drug targets that disrupt this process in patients.
The link between the nervous system and cancer progression has been recognized for years. One well-known example is “neural invasion,” a process in which nerve fibers from nearby healthy tissue grow into tumors. This phenomenon is commonly associated with poorer outcomes and more aggressive disease.
About six years ago, scientists in the United States reported a surprising finding in brain tumors. They showed that some cancers can form their own synapses, allowing them to directly exploit neuronal signaling. Inspired by this work, Professor Ekin Demir, a clinician scientist in the Department of Surgery at the TUM University Hospital, and his team set out to investigate whether tumors outside the brain might use a similar tactic.
Searching for “tumor synapses”
Pancreatic tumors were an obvious candidate because neural invasion is especially common in this cancer type. The researchers analyzed pancreatic tumor samples, looking for clusters of receptors that interact with specific neurotransmitters.
In several samples, they found elevated levels of NMDA receptors, which bind glutamate. Further analysis using electron microscopy revealed structures that closely resemble synapses. Because these formations differ in important physiological details from typical neuronal synapses, the team refers to them as pseudosynapses.
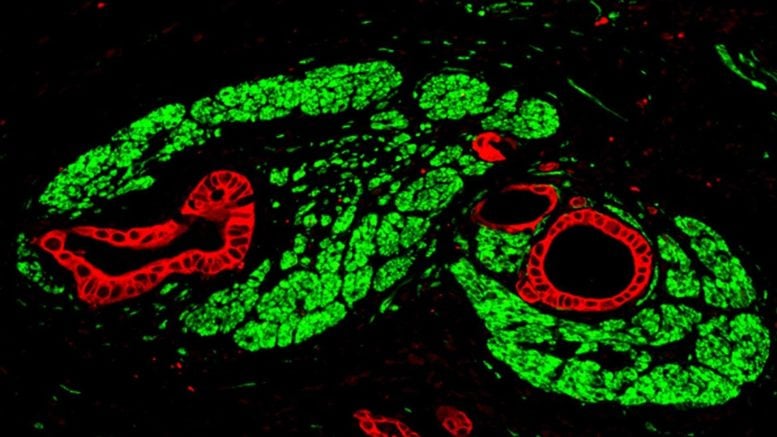
Calcium waves promote tumor growth
What advantage do pancreatic tumors gain by forming pseudosynapses? Like other glands, the pancreas is regulated by the nervous system. Depending on the body’s needs, healthy pancreatic cells receive the neurotransmitter glutamate through their synapses. This triggers a series of processes. Pseudosynapses exploit this natural mechanism.
“When glutamate binds to the cancer cells’ NMDA receptors, a channel opens, and calcium flows into the cell,” explains Professor Demir. “This influx triggers molecular signaling cascades that drive tumor growth and metastasis.” The team observed that the cancer cells generate characteristic slow, long-lasting calcium waves that drive tumor growth in a sustained way.
Yet this remarkable mechanism may open up a path to new cancer therapies. In mouse experiments, the researchers successfully blocked the NMDA receptors on tumor cells with a drug. The result: pancreatic tumors grew more slowly, developed fewer metastases, and the animals lived longer.
“We are currently using bioinformatic methods to identify approved drugs that, in addition to their primary effects, can also block these specific NMDA receptors in pancreatic cancer cells,” says Professor Ekin Demir. “Therapies targeting the interface between the nervous system and tumors could open up entirely new treatment options.” The team suspects that other tumor types may also form pseudosynapses to accelerate their growth.
Reference: “Sensory neurons drive pancreatic cancer progression through glutamatergic neuron-cancer pseudo-synapses” by Lei Ren, Chunfeng Liu, Kaan Çifcibaşı, Markus Ballmann, Gerhard Rammes, Carmen Mota Reyes, Sergey Tokalov, Andreas Klingl, Jennifer Grünert, Keshav Goyal, Peter H. Neckel, Ulrich Mattheus, Benjamin Schoeps, Saliha Elif Yıldızhan, Osman Ugur Sezerman, Nedim Can Cevik, Elif Arik Sever, Didem Karakas, Okan Safak, Katja Steiger, Alexander Muckenhuber, Kıvanç Görgülü, Zongyao Chen, JingCheng Zhang, Linhan Ye, Mohammed Inayatullah Maula Ali, Vijay K. Tiwari, Nataliya Romanyuk, Florian Giesert, Dieter Saur, Roland Rad, Roland M. Schmid, Hana Algül, Achim Krüger, Helmut Friess, Güralp O. Ceyhan, Rouzanna Istvanffy and Ihsan Ekin Demir, 25 September 2025, Cancer Cell.
DOI: 10.1016/j.ccell.2025.09.003
I.E.D. and L.R. were supported by a grant of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID DE 2428/11-1. I.E.D. was supported by the Else Kröner Clinician Scientist Professorship Program of the Else Kroner-Fresenius Foundation.
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.