What You Ought to Know:
– The U.S. Food and Drug Administration (FDA) has not too long ago issued a number of key clearances, marking important developments throughout diagnostics, surgical procedure, and distant affected person monitoring.
– These FDA clearances mirror a deepening integration of Artificial Intelligence (AI) and superior know-how into scientific workflows, promising higher precision, lowered invasiveness, and expanded entry to care.
Diagnostics and Distant Monitoring: AI and Wearables at Scale
A serious development in latest clearances focuses on leveraging AI and comfy wearables to reinforce diagnostic velocity and continuity of care.
Wearable Cardiopulmonary Monitoring
Hexoskin Receives 510(okay) Clearance for Lengthy-Time period ECG and Respiratory Monitoring

Carré Technologies Inc. (dba Hexoskin) received 510(okay) clearance for its Hexoskin Medical System (HMS) for steady long-term ECG, respiratory monitoring, and exercise in ambulatory sufferers. This technique, which features a sensible biometric shirt, turns into one of many first medical-grade wearable programs able to long-term ECG and respiratory measurements exterior the clinic, remodeling distant care and decentralized scientific trials. The know-how permits physicians to evaluate arrhythmias (like atrial fibrillation) and respiratory fee patterns with steady information assortment.
For scientific analysis, HMS represents a serious step ahead. With FDA clearance, the system can now assist decentralized trials, permitting investigators to seize high-resolution, real-world physiological information and develop AI-driven digital biomarkers throughout cardiology, pulmonology, neurology, and uncommon illnesses.
AI-Pushed Cardiac and Aortic Imaging
FDA clearances solidify AI’s function in cardiovascular danger administration:
RapidAI Receives FDA Clearance for Speedy Aortic, Bringing Deep Medical AI to Aortic Illness Administration
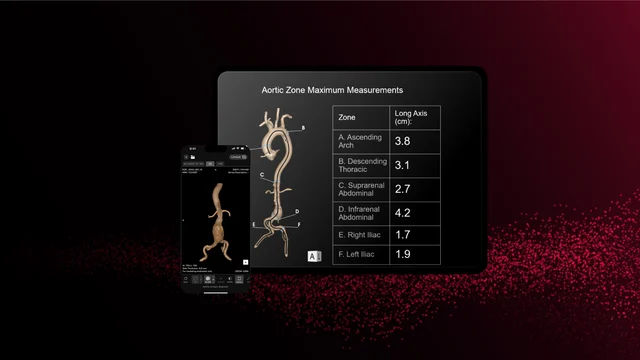
RapidAI earned FDA clearance for Aortic Administration, a part of its Speedy Aortic product. This deep scientific AI answer transforms the acute evaluation and longitudinal administration of aortic illness. It mechanically generates essential measurements (together with zonal maximums and landmark metrics), produces 3D reconstructions, and tracks anatomical adjustments over time to help in figuring out and monitoring pathology from the aortic arch to the iliacs.
Not like conventional AI triage instruments, Speedy Aortic is engineered to assist end-to-end affected person administration: screening, prognosis, therapy planning, and surveillance. The system’s potential to course of all CT scans containing the aorta—whether or not distinction or non-contrast—expands its utility throughout emergency, inpatient, and outpatient settings.
Clinicians stand to profit from lowered cognitive burden, sooner learn occasions, and improved accuracy. Surgeons can leverage exact visualizations for pre-procedural planning, whereas well being programs achieve a unified workflow built-in via the Speedy Edge Cloud and Speedy Navigator Professional.
Circle CVI Receives 510(okay) Clearance for AI-Enabled Coronary Plaque Evaluation
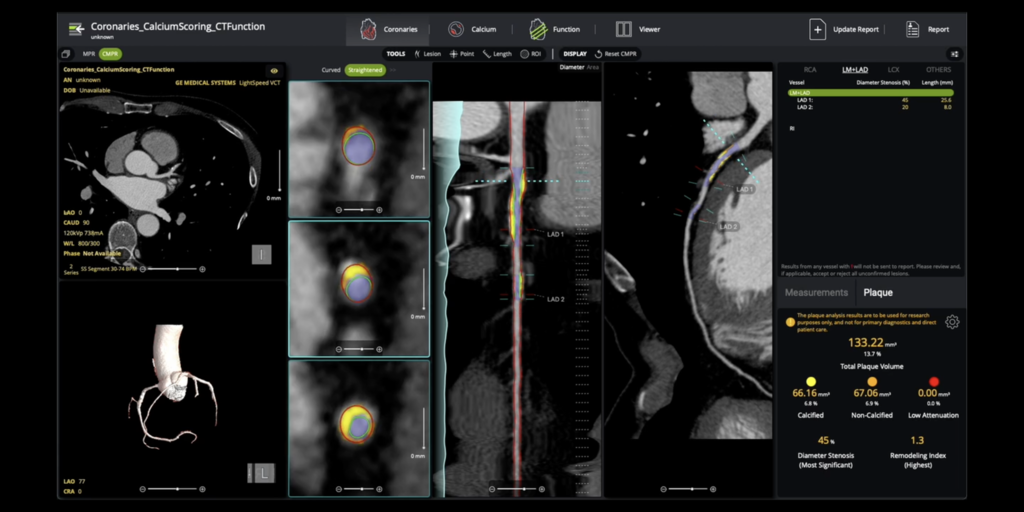
Circle Cardiovascular Imaging Inc. (Circle CVI) obtained 510(okay) clearance for its cvi42 | Plaque answer for complete coronary plaque evaluation. This AI-enabled, on-premise know-how quantifies whole, calcified, and non-calcified plaque, supporting exact danger stratification. The clearance coincides with a brand new Class I CPT code (75XX6) taking impact in January 2026, solidifying plaque quantification as commonplace scientific care.
Bunkerhill Well being Secures FDA Clearance for First AI to Detect Mitral Annular Calcification on Routine Chest CT

Bunkerhill Health achieved the first-ever FDA clearance for an AI algorithm—Bunkerhill MAC—to detect and quantify mitral annular calcification (MAC) on routine, non-gated chest CT scans. MAC is an often-overlooked discovering linked to elevated cardiovascular danger and procedural issues.
Built-in into Bunkerhill’s Carebricks platform, the software leverages FDA-cleared AI and large-language-model-based reasoning to assist follow-up choices inside cardiology, major care, and structural coronary heart packages. The clearance displays the FDA’s broader confidence in AI that elevates incidental findings into actionable scientific insights.
Neuroscience and Ache Administration
A number of clearances goal mind perform and continual ache:
QuantalX Secures De Novo Clearance for Delphi-MD, a First-of-Its-Type Purposeful Neuro-Imaging Know-how
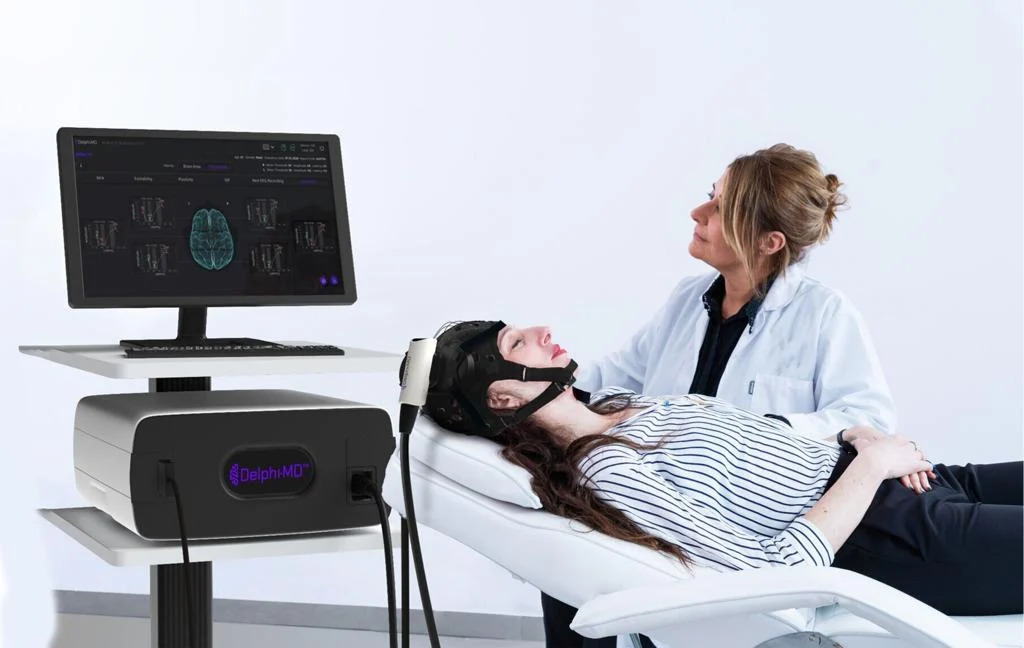
QuantalX Neuroscience was granted De Novo classification for its Delphi-MD™ System, establishing a brand new modality of useful neuro-imaging (FNI). Delphi-MD combines non-invasive Transcranial Magnetic Stimulation (TMS) with electroencephalography (EEG) to benchmark mind community perform in opposition to a normative database.
Delphi-MD provides physicians a benchmarked evaluation of mind perform utilizing an FDA-cleared normative database of wholesome adults. This creates a novel scientific modality able to monitoring cognitive decline, evaluating neurological interventions, and aiding illness administration throughout neurodegenerative, traumatic, or pain-related circumstances.
Magstim Achieves FDA Clearance for Non-Invasive Magnetic Stimulation for Power Ache
The FDA cleared Magstim Magnetic Stimulation for the therapy of continual ache, offering a clinically confirmed, non-invasive, and drug-free possibility. The know-how modulates peripheral nerve pathways utilizing magnetic pulses, reaching deeper nerves with out invasive implants or prescription drugs.
Magstim’s know-how—cited in additional than 15,000 scientific research—gives deeper nerve stimulation in contrast with conventional TENS or surface-level gadgets, providing an essential possibility for sufferers for whom typical therapies are insufficient.
Surgical Robotics and Precision Orthopedics
Improvements in robotic help purpose to enhance precision and cut back trauma:
Zimmer Biomet Secures 510(okay) Clearance for ROSA Knee With OptimiZe, Increasing Robotic Precision in Orthopedics
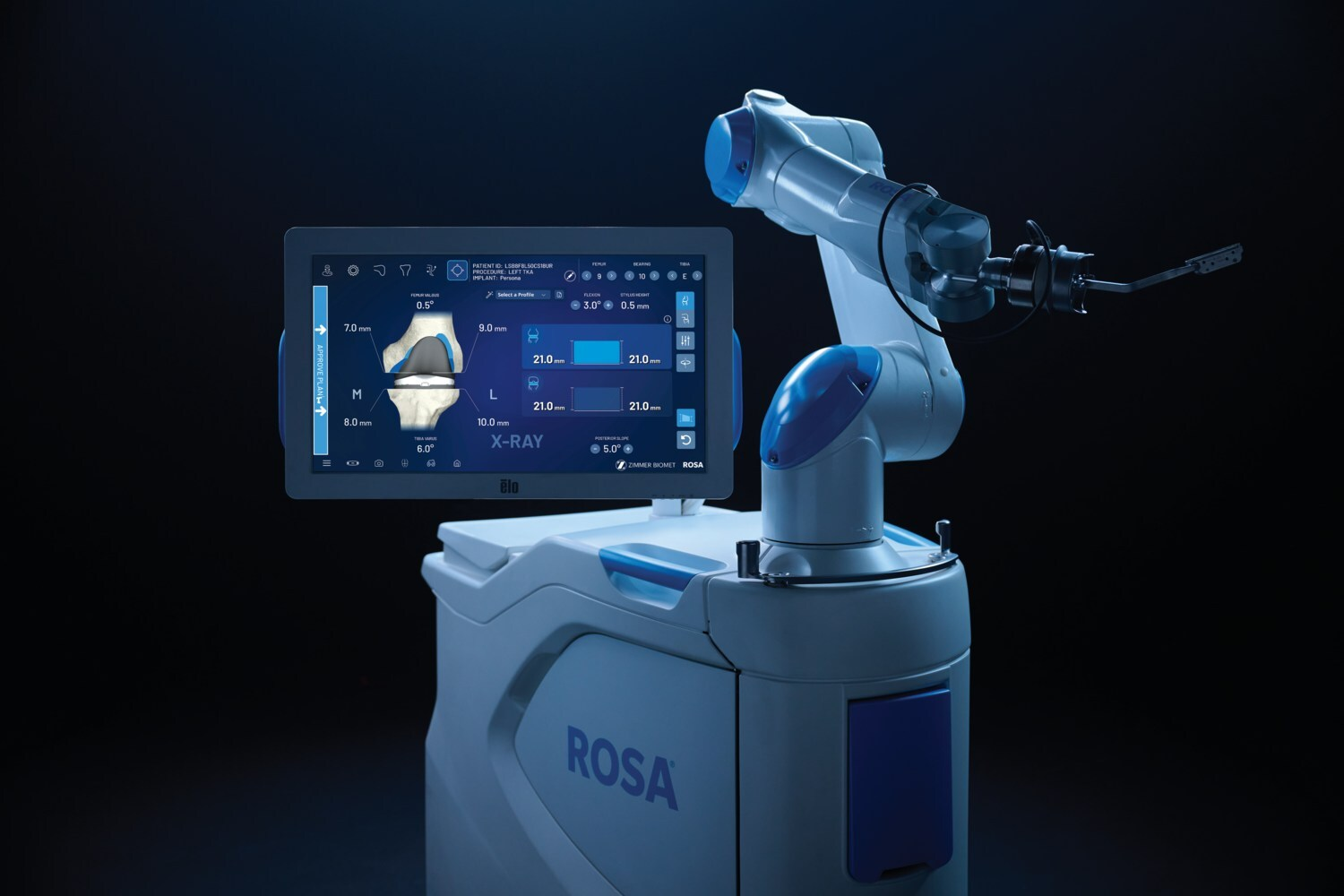
Zimmer Biomet gained 510(okay) clearance for ROSA® Knee with OptimiZe™, an enhanced model of its robotic system for whole knee substitute surgical procedure. The know-how presents personalized clever surgical planning and options like OptimiZe Kinematic Alignment™—the trade’s solely automated kinematic alignment characteristic—to make sure correct, reproducible outcomes based mostly on affected person anatomy and surgeon preferences.
Built-in with ZBEdge® Analytics, ROSA Knee with OptimiZe permits data-driven choices, real-time intraoperative insights, and steady efficiency analysis. A focused launch will start later this yr, with U.S. business availability anticipated in early 2026.
Levita Magnetics Beneficial properties Pediatric Clearance for Magnetic Surgical System (MSS)
Levita® Magnetics achieved FDA clearance for its Magnetic Surgical System (MSS) for pediatric sufferers. This know-how makes use of exterior magnets to regulate inside surgical devices, decreasing the variety of incisions wanted for procedures like laparoscopic cholecystectomy, which is essential for minimizing trauma and scarring in youngsters.
Cleveland Clinic Kids’s turned the primary middle to carry out a pediatric case utilizing this know-how. For youthful sufferers, minimizing tissue trauma is essential: fewer ports can result in sooner restoration, decreased ache, lowered scarring, and decrease complication dangers.
FDA Approves First-Ever Robotic Surgical Research for Alzheimer’s Illness Intervention

MMI (Medical Microinstruments, Inc.) obtained FDA Investigational Gadget Exemption (IDE) approval for a scientific examine—REMIND—utilizing the Symani® Surgical System for a novel microsurgical intervention for Alzheimer’s illness. The examine goals to reestablish lymphatic drainage pathways within the deep cervical lymph nodes to probably enhance the clearance of dangerous proteins. The process requires supermicrosurgical precision, working on vessels as small as 0.2mm.
Wound Care and Diabetes Administration
Speedy Nexus Earns 510(okay) Clearance for Hemastyl, a Breakthrough in Power Wound Remedy
Rapid Nexus Nanotech Wound Solutions, Inc. obtained FDA 510(okay) clearance for its Hemastyl gel system. The gel is the primary system to immediately deal with the periwound tissue setting—the dwelling edge chargeable for stalled restoration—to revive circumstances for wound closure and assist sufferers keep away from amputations.
Speedy Nexus plans to pursue FDA Breakthrough Gadget Designation, which may expedite protection and reimbursement pathways, making this remedy out there to tens of millions of high-risk sufferers nationwide. The clearance is a landmark second for wound therapeutic—a discipline lengthy dominated by symptomatic slightly than causative therapy approaches.
Tandem Diabetes Care Beneficial properties FDA Clearance for Tandem Mobi App for Android Customers
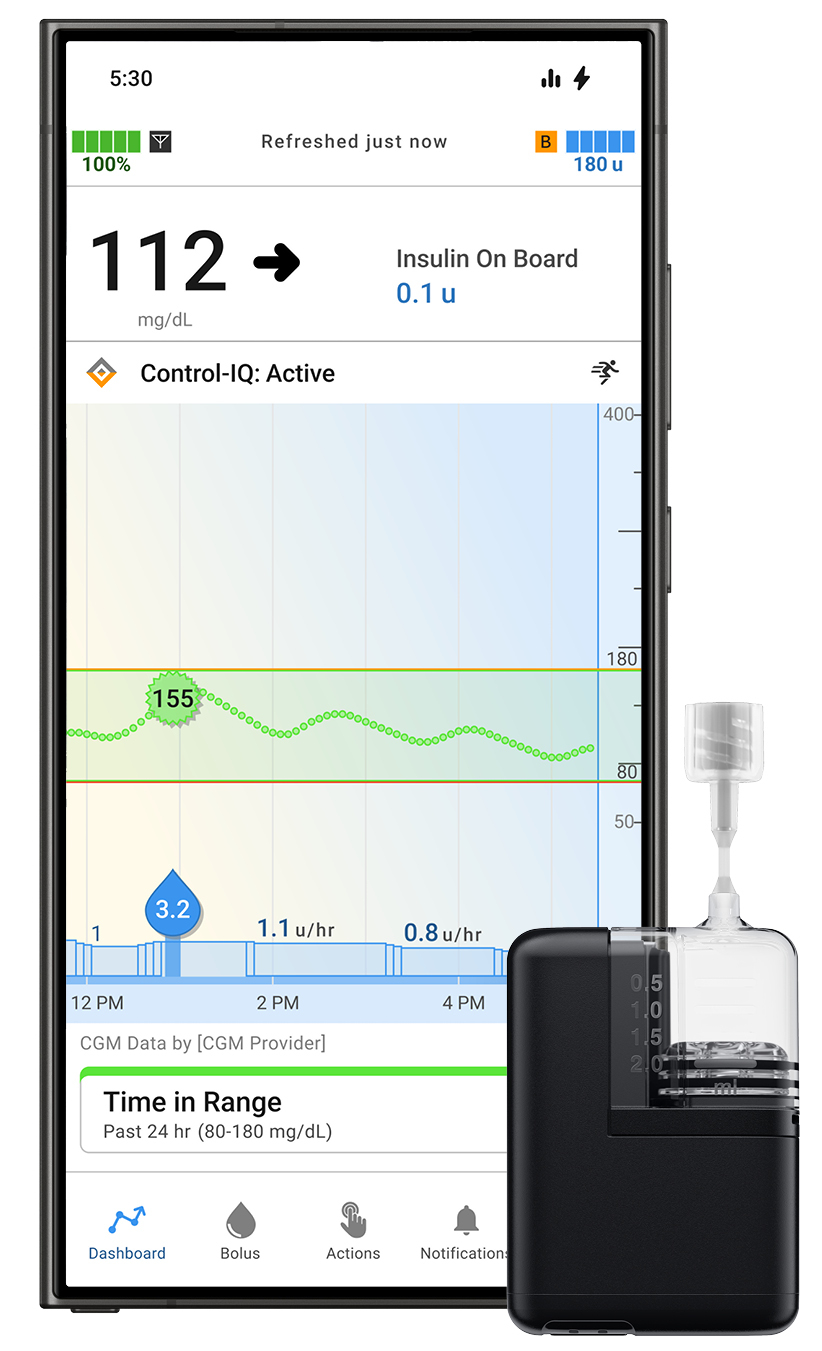
Tandem Diabetes Care, Inc. obtained FDA clearance for the Android model of its Tandem Mobi cell app. This enables Android customers to handle their diabetes immediately from their suitable smartphone utilizing the Tandem Mobi automated insulin supply system, which is powered by Management-IQ+ know-how.
The clearance considerably expands entry for sufferers preferring Android gadgets, bettering usability and affected person adherence. A restricted launch is predicted in late 2025, with broad business availability in early 2026.